Testresults 1
- the silverfibre is an approved antimicrobial agent and registered with the EPA.
- the silverfibre is used in many FDA approved products, ranging from bandages to Class II medical devices used in corneal transplants.
The socks with silverfibre are Lauer listed EU Medical Products Class I with PZN (pharmaceutical central no.) and may be prescribed. They are textiles, produced according to OEKOTEX 100 standard, free from harmful substances and have also been tested widely in Germany and other countries.
New York University, September 7th, 1997
Independent Third Party Testing
Dr. Philip Tierno
This study utilized the traditional Kirby-Bauer method for determination of antimicrobial efficacy. The results confirm not only the excellent performance of the silverfibre as an antimicrobial agent but also show that the fabric tested was effective in killing MULTIDRUG resistant bacteria. The testing confirms that the silverfibre is a viable and cost effective mechanism to address the growing problem of antibiotic resistant microbes.
NAMSA
Independent Third Party Testing
United States of America
NAMSA is one of the most well respected independent laboratories in the United States. It is both EPA and FDA certified. These numerous tests verify the exceptional performance of the silverfibre. Of note in these studies is that they indicate that the silverfibre is a broad-spectrum antibacterial agent. NAMSA testing also indicates the exceptional performance of the silverfibre as an anti-fungal; specifically against T. Mengatrophytes the fungus which causes Athlete’s Foot.
Tokai University, May 1997
Independent Third Party Testing
Dr. Seiki Tazume and Dr. Takahiko Yoshida
This study conducted in Japan demonstrated in a real world setting, consisting of 20 volunteers, that the silverfibre demonstrated an excellent reduction in bacterial and fungal counts. These results coincide with the rapid development of the Japanese hosiery market. the silverfibre is widely recognized in Japan as the most effective antimicrobial alternative. The silverfibre is approved by the Ministry of Health in Japan as an antimicrobial agent and exceeds the industry minimum standards of performance.
TÜV Rheinland Product Safety, ©2005
General requirement acc. DIN EN 61340-5-1,
Test acc. DIN EN 1149-2
Resistivity of these socks is remarkably below the required electrical resistance. The silvertextiles are suitable for EPA environments.
Testresults 2
Textilresearch Institute Thüringen -Vogtland e.V./Germany/2003
Textilephysical properties of BEST4FEET Diabetic socks without rubber
Scrubbing, fitting change, resistance to perspiration. Measure of pressure at the top of the socks results in only 3,5 mmHg!
Pennsylvania State University, ©1987
Silver-Coated Nylon Fiber as an Antibacterial Agent
Antimicrobial Agents and Chemotherapy - American Society of Microbiology
This published and peer reviewed study definitively proved the antibacterial properties of the silverfibre. This analysis also verified that an extract derived from the fiber was bactericidal. This attribute explains the disinfecting quality witnessed by the wearing of hosiery containing the silverfibre. Of particular interest, the efficacy of the silverfibre was demonstrated to be more bactericidal than silver nitrate.
Pennsylvania State University, ©1987
Effect of Silver Coated Thread on the Microbial Population of Shoes
“Interest in the project developed from our previous studies, from which we found that silver coated thread (the silverfibre) was remarkably inhibitory to the growth of and reproduction of several human pathogenic bacteria. Obviously, the presence of silver in the right shoe of the volunteers greatly diminished the total count of microorganisms and their associated odors.”
Cornell University, ©1987
Newly Made Antibacterial Braided Sutures: In Vitro and In Vivo Biocompatibility Study
Journal of Biomedical Materials Research
This published and peer reviewed study demonstrates antibacterial properties toward new and established bacterial colonies. Additionally, the biocompatibility data suggested that the silverfibre fiber implanted into gluteal muscle caused less of an inflammatory reactionthan that of ordinary nylon. This study reaffirms the intrinsic safety of the silverfibre.
Scientific Publications
Silver-Coated Nylon Fiber as an Antibacterial Agent
Patricia C. MacKeen, Stanley Person, Susan C. Warner, Wallace Snipes, Edward Stevens Jr
from : American Society of Mircobiology, Januar 1987, Seite 93-99
In Vitro Quantitative Study of newly made Antibacterial Braided Nylon Structures
W.C. Tsai, PH.D, Taipei/Taiwan, C.C.Chu, PH.D. Ithaca, New York a.o.
from: SURGERY, Gynecology & Obstetrics, September 1987, Vol. 165, 207-211
Testresults 3
TOKAI University, Japan, May 1997
Independent Test conducted by: Dr. S. Tazume, Dr. T. Yoshida
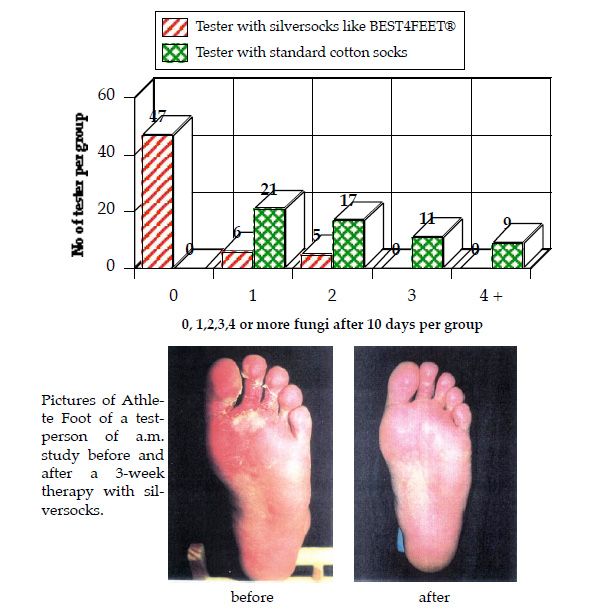
Testdescription: one group of testpersons wear standard cotton socks for 3 days, then for 8 days the silversocks (8-12 hrs/day), the second group wear only cotton socks. Bacteria and fungi were counted on day 12.
95% of testpersons with standard cottons socks showed 30 or more staphylococcus epidermis. 100% show one or more fungi!
In comparison: 92% of the group with the silversocks were absolutely free of bacteria, respectively 78% free of fungi.
The socks with silverfibre are therefore a proven product to fight Athlete Foot and bacteria causing odours.